


Ecotoxicology and Environmental Safety is a multi-disciplinary journal that focuses on understanding the exposure and effects of environmental contamination on organisms including human health. The scope of the journal covers three main themes. The topics within these themes are indicated (but are not limited to) Ecotoxicology, Environmental Chemistry and Environmental Safety.
On September 15, 2021, Professor Zhang Huiping's research team from the Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, published a paper titled "Cardiac developmental toxicity and transcriptome analyses of zebrafish (Danio rerio) embryos exposed to Mancozeb" on the online journal Ecotoxicology and Environmental Safety,in which molecular mechanism of zebrafish heart developmental injury induced by dysenzin-manganese (MZ) exposure was revealed.
Mancozeb (MZ), an antibacterial pesticide, has been linked to reproductive toxicity, neurotoxicity, and endocrine disruption. However, whether MZ has cardiactoxicity is unclear. In this study, the cardiotoxic effects of exposure to environment-related MZ concentrations ranging from 1.88 μM to 7.52 μM were evaluated at the larval stage of zebrafish. Transcriptome sequencing predicted the mechanism of MZ-induced cardiac developmental toxicity in zebrafish by enrichment analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO). Consistent with morphological changes, the osm, pfkfb3, foxh1, stc1, and nrarpb genes may effect normal development of zebrafish heart by activating NOTCH signaling pathways, resulting in pericardial edema, myocardial fibrosis, and congestion in the heart area. Moreover, differential gene expression analysis indicated that cyp-related genes (cyp1c2 and cyp3c3) were significantly upregulated after MZ treatment, which may be related to apoptosis of myocardial cells. These results were verified by real-time quantitative RT-qPCR and acridine orange staining. Our findings suggest that MZ-mediated cardiotoxic development of zebrafish larvae may be related to the activation of Notch and apoptosis-related signaling pathways.
Master Wang Yongfeng from the Institute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology is the first author of the paper, and Professor Zhang Huiping and Lecturer Liu Chunyan are the corresponding authors of the paper. This research was funded by the National Key R&D Project (2018YFC1004300,2018YFC1004304).
The result pictures below are obtained from the paper (filmed by Micro-shot camera MS23, under Micro-shot upright fluorescence microscope MF43-N)
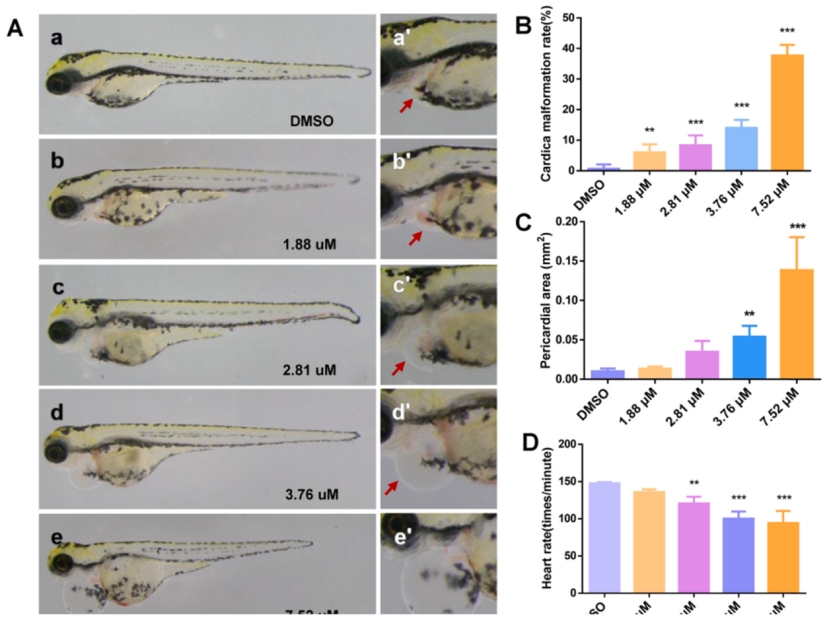
Fig. 1. Phenotype of MZ-treated zebrafish larvae. (A) a–e is the heart at 2.5× magnification, and a '-e' is the heart at 5.0× magnification. (B–D) Malformation rate, pericardial edema, and heart rate of zebrafish larva heart, respectively. The pericardium is indicated by the red arrow. An asterisk (*) indicates a significant difference (p ) between the treatment groups and the control group (**p ; ***p ). n = 15 larvae per group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
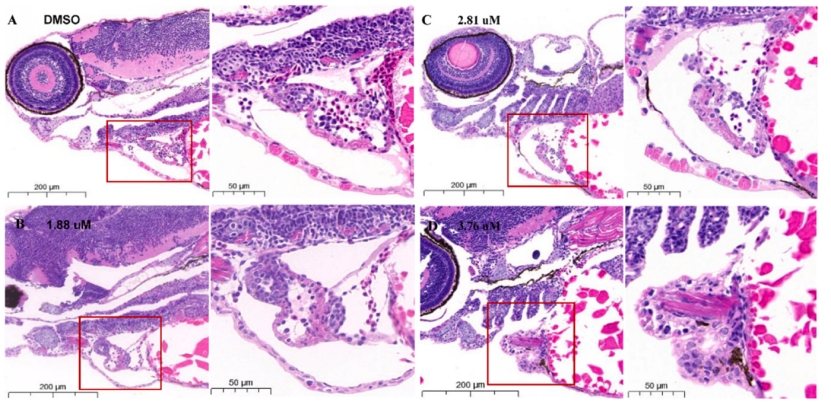
Fig. 2. Representative images reflecting the effects of different MZ concentrations on heart tissues in zebrafish at 96 hpf. n = 15 larvae per group.
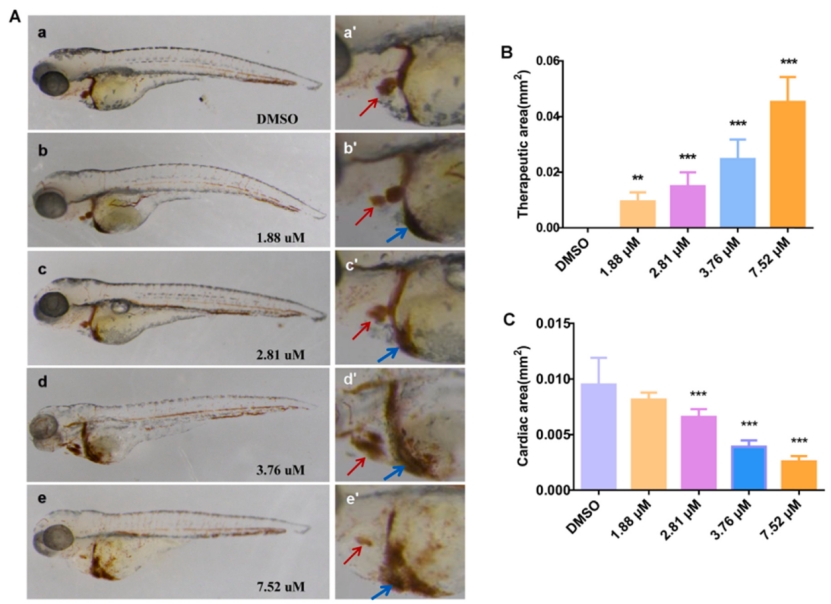
Fig. 3. Effects of exposure to different MZ concentrations at 96 hpf on the cardiac developmental toxicity of zebrafish. (A) a–e is the heart at 2.5× magnification, and a '-e' is the heart at 5.0× magnification. The atrium and the ventricle are indicated by the red arrow, whereas the area of congestion is indicated by the blue arrow. (B) Results of quantitative statistical analysis of cardiac congestion in each concentration group. (C) Results of quantitative analysis of ventricle and atrium area in each concentration group. An asterisk (*) indicates a significant difference (p ) between the treatment groups and the control group (**p ; ***p ). n = 15 larvae per group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
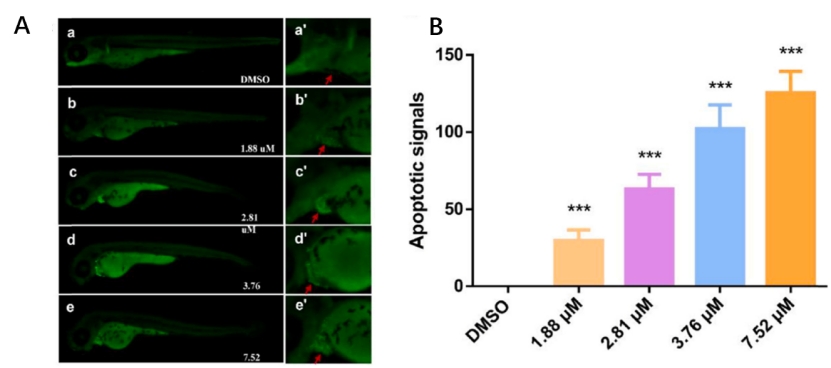
Fig. 4. MZ induced apoptosis in the zebrafish larvae at 96 hpf as detected by acridine orange (AO) staining. The red arrow marks the apoptotic signal. (D) Relative fluorescence of region with bright spots in AO staining as quantified using Image J. An asterisk (*) indicates a significant difference (p ) between the treatment groups and the control group (**p ; ***p ). n = 15 larvae per group.

The phenotypes of zebrafish’s pericardial edema under different exposure concentrations.
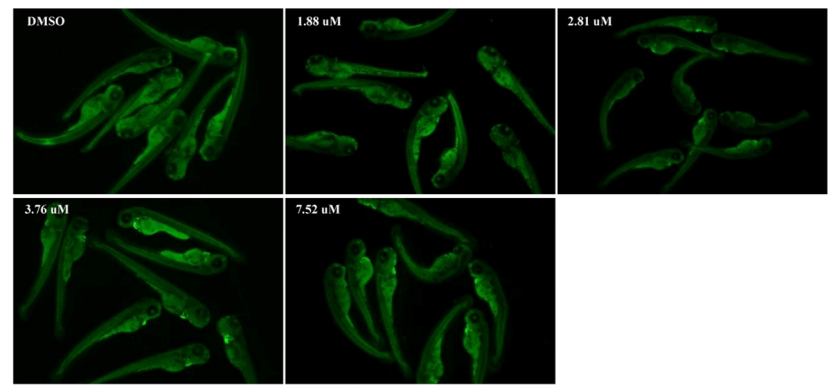
Fig. A3. The signal of cardiomyocyte apoptosis under different MZ concentrations. The heart was magnified 2 times.
