Fluorescence imaging brings infinite possibilities to life science research. From wide-field fluorescence to confocal to super-resolution, the accuracy of our observation is constantly improving, but one problem always exists – fluorescence quenching. Today we will take a look at the tool paper of “Nature” magazine, “Towards Brighter and More Light-Stable Fluorophores”, and learn how to make more light-stable fluorophores.
I、 What is fluorescence quenching?
First define fluorescence quenching. Fluorescence quenching, or more commonly known as “fading”, refers to any process that reduces the fluorescence intensity of a fluorophore.
①It can be roughly divided into four types:
1.Complex formation
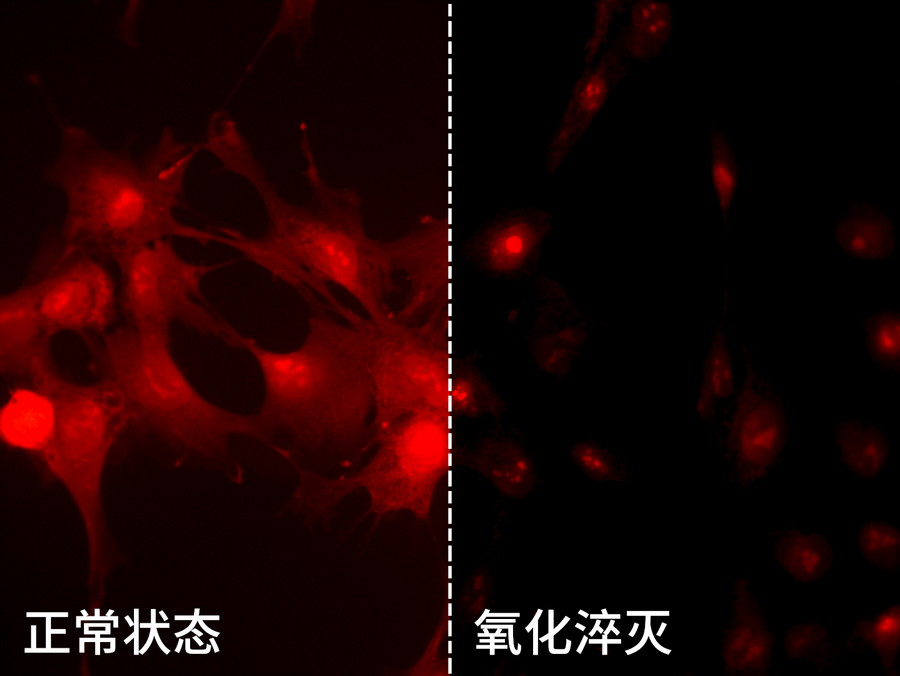
Fluorescent substance molecules react with external molecules/ions to form non-fluorescent substances. Common fluorescence quenching is oxidation under high light intensity to form new compounds. This phenomenon is also called photo bleaching. It is irreversible damage.
2. Energy transfer
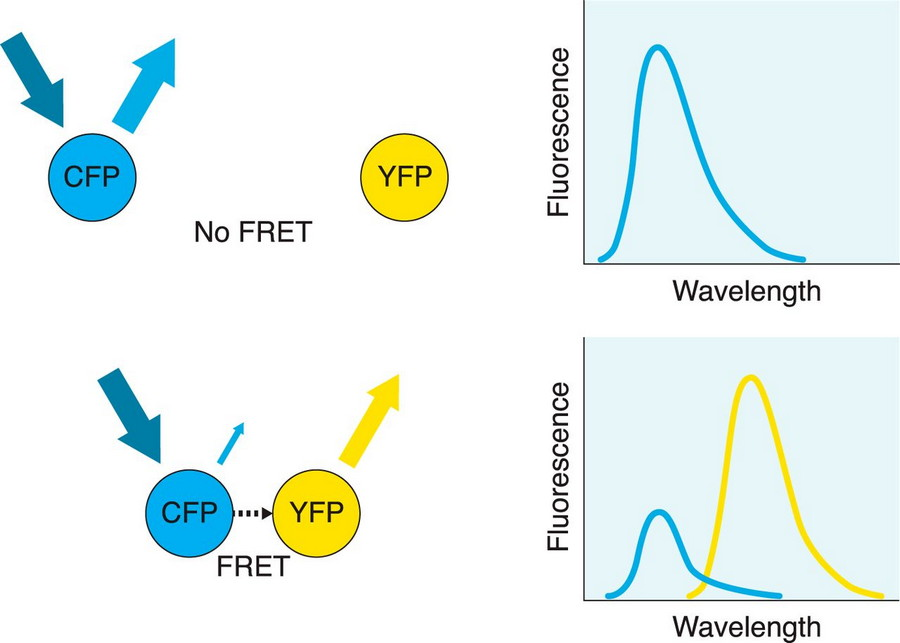
As the name suggests, there is a transfer of energy from the donor to the acceptor. If the acceptor does not produce fluorescence, the fluorescence is quenched: if the acceptor generates fluorescence, there will be a red shift of the acceptor fluorescence and secondary fluorescence spectra, which is Fluorescence Resonance Energy Transfer FRET.
3.Excited state reactions
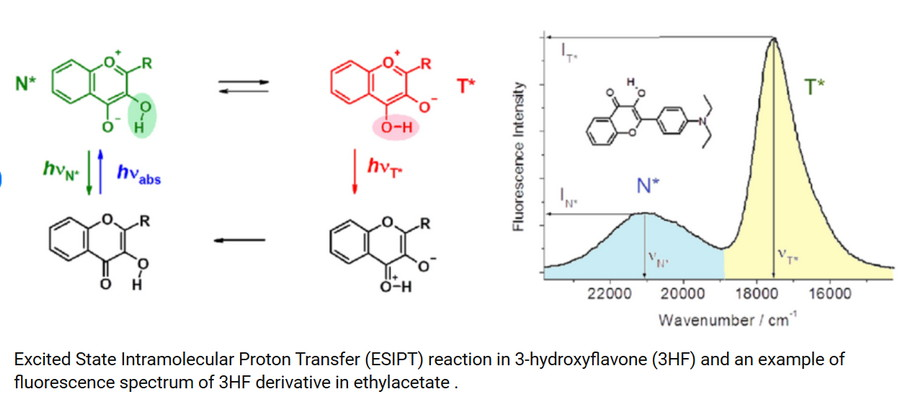
Under high energy in the excited state, the chemical structure of the fluorophore undergoes structural destruction and changes the fluorescence characteristics. This phenomenon is called excited state intramolecular proton transfer ESIPT, which can be understood as the proton transfer version of 2.
4. Collisional quenching
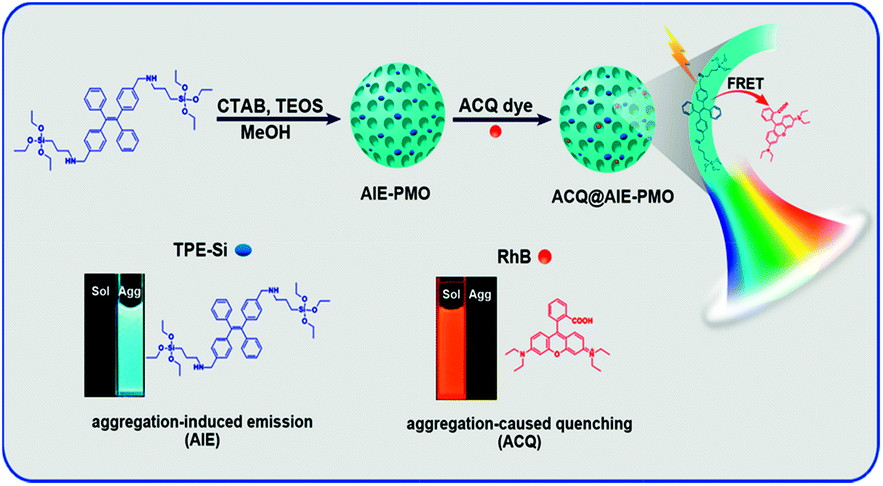
The aggregation or too close distance of fluorescent molecules causes mutual interference, increases the probability of non-radiative attenuation, and decreases the luminous efficiency. This phenomenon is called aggregation inducement quenching ACQ. Some fluorophores, in turn, enhance fluorescence when they are in an aggregated state. This phenomenon is aggregation-induced fluorescence emission (AIE).
Two、three attempts to transform fluorophores④
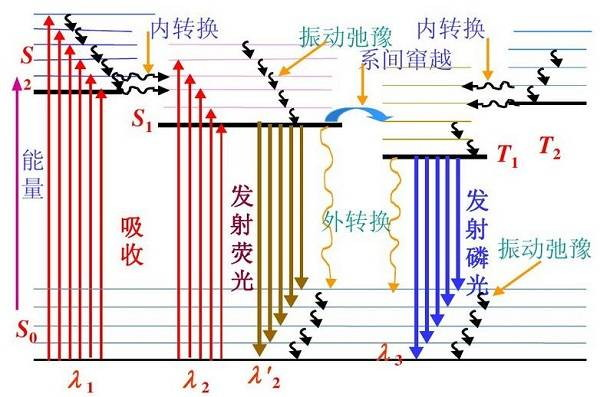
In order for the fluorophore to return to the normal state from the excited state, it needs to release energy, either through fluorescence or non-radiative attenuation in the form of vibration or other forms. Theoretically, reducing the proportion of non-radiative attenuation can increase the proportion of fluorescence release, thereby obtaining higher light intensity and longer fluorescence lifetime.
a. strengthen mCherry of protein structure
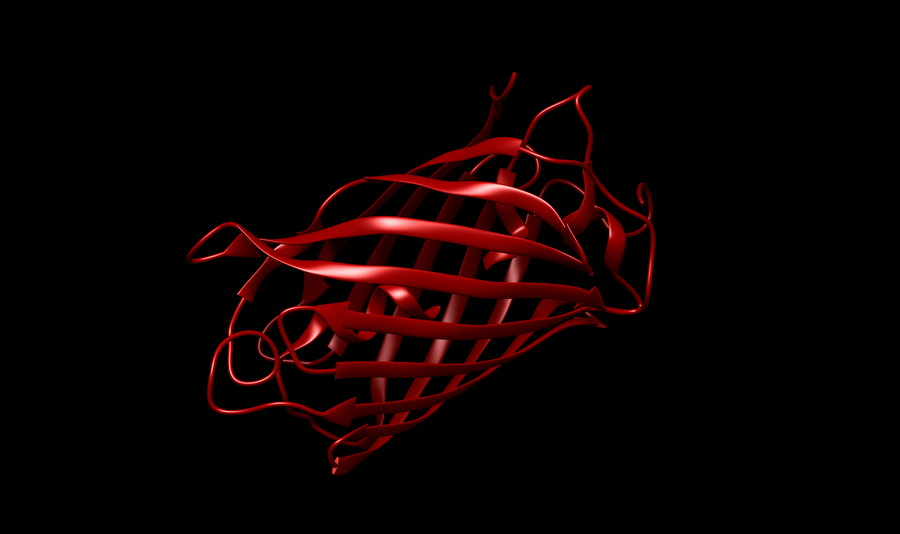
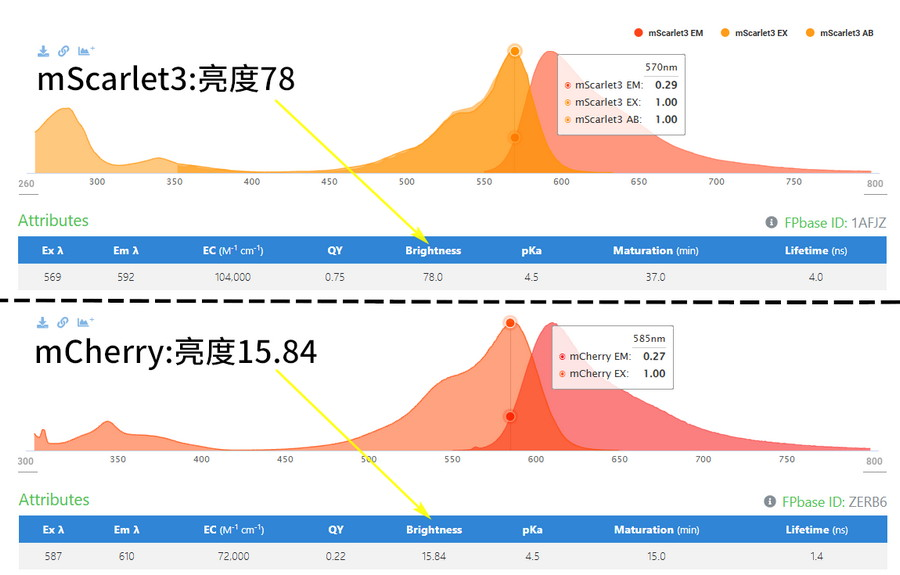
By changing the protein scaffold structure of the fluorescent protein to make it more stable, thereby increasing the fluorescence intensity and extending the fluorescence lifetime, such as “reinforcing” the mcheny-barrel structure to prevent the molecular rearrangement of the chromophore, non-radiative attenuation can be reduced, and both mChery-XL and mScarlet3 are brighter and more photostable than mCherry.
b.Turn off non-radiative attenuated EGFP
Through simulation calculations, scientists found that a non-radiative pathway for EGFP is the transfer of electrons from the chromophore to the tyrosine residue, thereby changing the fluorescence properties. Replacing tyrosine with leucine can block this non-radiation pathway and successfully increase the photostability of EGFP by 80 times. Unfortunately, the brightness of this EGFP is negatively affected and becomes darker, so it may not be useful in the biological field.
c.Improved industrial rhodamine series dyes
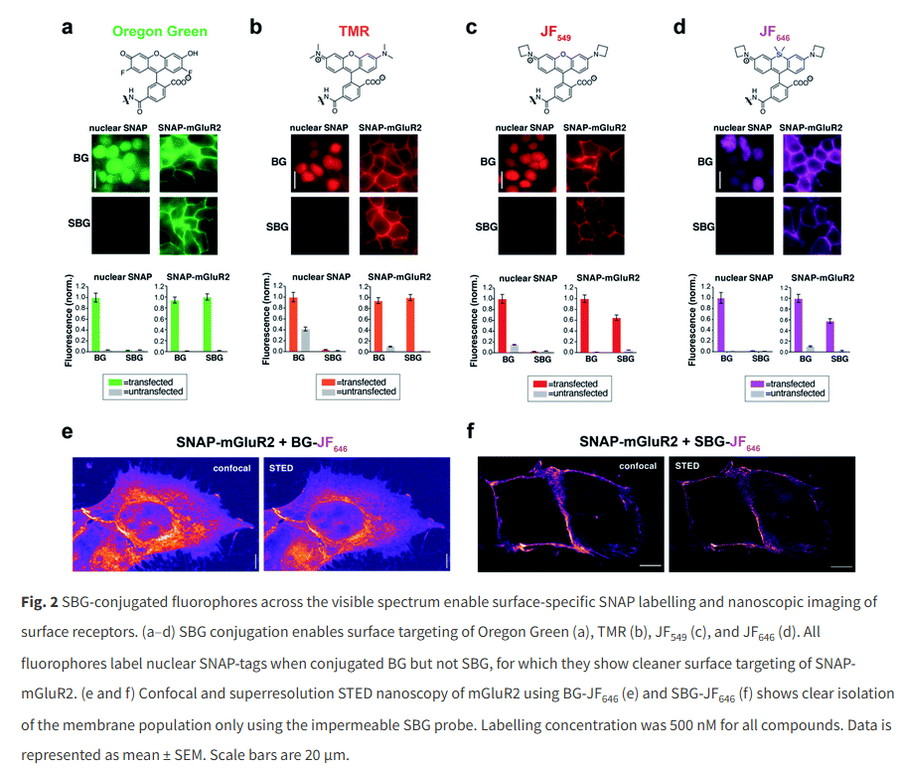
Rhodamine is the basic component of Texas Red, ROX, TAMRA and other dyes. The traditional synthesis process has limitations in terms of reagent environment and precision control. Some scientists have successfully improved it with a more modern synthesis process. In the alkylamino substituent, the introduction of deuterium atoms changes key molecular dimensions, thereby reducing non-radiative attenuation. The result is the Janelia Huor series of fluorescent dyes, which cover the fluorescence spectrum from green to red and can provide fluorescence intensity and fluorescence lifetime several times that of traditional fluorescent dyes.
III. How to better use these improved fluorophores?

After the fluorophore is improved, not only the fluorescence intensity will be higher and the fluorescence lifetime will be longer, but its excitation and emission properties may also change. Such as mCherry-XL and mScarlet3, they need to be excited using the Y channel instead of the original mCherry’s R channel excitation.

MF43-N and MF53-N can support mChery-XL and mScaret3 by installing the Y excitation block/G2 excitation block, while other microscopes can choose the digital display fluorescence module with Y channel to achieve support.

But the greater value of these brighter and more photostable fluorophores lies in their suitability for higher-demand imaging, such as confocal and super-resolution fluorescence imaging. When using MF53-TIRF to upgrade to dSTORM imaging, you need to use high-brightness, high-light-fast fluorescent dyes to obtain imaging with a higher signal-to-noise ratio. In some neurological experiments, scientists have used these new dyes to realize fluorescent labeling imaging of millisecond-level electrical signal changes in brain nerves, thereby more deeply revealing the mechanisms of neuronal information processing and learning.
Article citation:
①Lakowiz, 1R.(1983). Quenching of Fluorescence.ln: prindiples of Fluorescence specroscopy, Springer Boston, MA., hps:l/doior/10.1 0071978-1-46157658-7 9
②pivovarenko, Vasyl. (2023). Mul-Parametric Sensing by Multi-channel Molecular fluorescent probes Based on Excted State lntramolecular protonTransfer and Charge Transfer Processes.BBAAdvances.3.100094.10.1016/jbbadva.2023.100094.g
③Dongdong L, Yuping Zhang, Zhying Fan , lie chen and jihong Yu, Coupling of chromophores wth exacly opposite luminescence behaviors inmesostructured organoslicas for high-efficiency multicolour emission,DOl: 10.1 039/c5$c02044A (Edge Artice) chem. d, 2015, 6, 6097-6101
④Aana REmmEI .TOWARDS BRIGHTER AND MORE PHOTOSTABLE FLUOROPHORES. Nstur, 630,258-260 (2024), doi: https:/dol.org/10.1038/d41586.024-01591-7
⑤Li Yingchun, molecular luminescence analysis of fluorescence phosphorescence, School of Pharmacy, Shihezi University.
⑥Pascal Poc ORclD logoa, et alinterrogating surface versus intracellular transmembrane receptor populations using cellimpermeable SNAp-tagsubstrates, DOl:10.1039/00$C02794D(Edge Artide) Chem.Sci. 2020 11 7871-7883